Facile synthesis of a zinc oxide nanoparticle by electrochemical method
DOI:
https://doi.org/10.11113/mjfas.v10n3.319Keywords:
Nanostructures, Chemical synthesis, Electron microscopy, Infrared spectroscopy, X-ray diffractionAbstract
An electrogenerated zinc oxide (EGZnO/Naph) nanoparticle was successfully synthesized by electrolysis of a N,N-dimethylformamide (DMF) solution containing naphthalene and a supporting electrolyte in a one-compartment cell fitted with a platinum cathode and a zinc anode. X-ray diffraction (XRD) and transmission electron microscopy (TEM) studies showed that the EGZnO/Naph consists of pure single crystalline wurtzite of hexagonal structure with average diameters of 10-15 nm. The BET surface area of the EGZnO/Naph was 65 m2/g, which is 15 times larger than that of commercial ZnO powder. The zinc oxide was also confirmed by Fourier transform infrared (FTIR) results which showed vibrational bands at 500 and 434 cm−1. Furthermore, the absorption peak of the EGZnO/Naph obtained at 366 nm (3.35 eV), is very close to the band gap of the ZnO 1s–1s electron transition (3.37 eV). Based on these results, this study reports a new pathway to synthesize nanosize of ZnO particle using a simple electrochemical process.
________________________________________
GRAPHICAL ABSTRACT
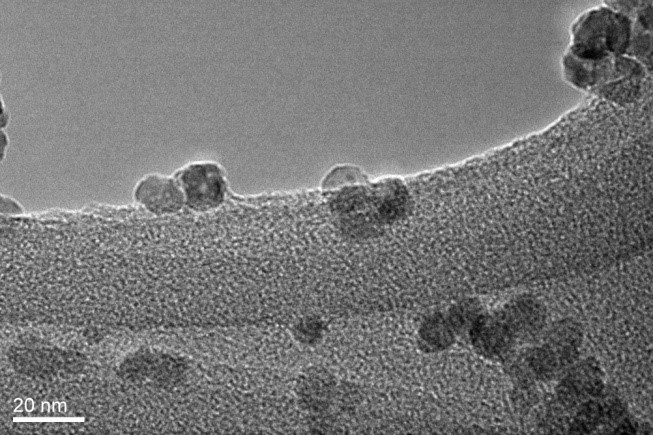
References
N. K. Park, Y. J. Lee, S. H. Yoon, G. B. Han, S. O. Ryu, T. J. Lee, W. G. Lee, Y. J. Bae, Scripta. Mater. 59 (2008) 328.
K. Ramamoorthy, M. Arivanandhan, K. Sankaranarayanan, C. Sanjeeviraja, Mater. Chem. Phys. 85 (2004) 257.
Y. Gui, C. Xie, Q. Zhang, M. Hu, J. Yu, Z. Weng, J. Cryst. Growth. 289 (2006) 663.
M. G. Ma, Y. J. Zhu, G. F. Cheng, Y. H. Huang, Mater. Lett. 62 (2008) 507.
J.B. Reitz, E.I. Solomon, J. Am. Chem. Soc. 120 (1998) 11467.
N. Takahashi, Mater. Lett. 62 (2008) 1652.
Z. Yang, C. Luan, W. Zhang, A. Liu, S. Tang, Thin Solid Film. 516 (2008) 5974.
A. E. Kandjani, M. F. Tabriz, B. Pourabbas, Mater. Res. Bull. 43 (2008) 645.
M. Sun, W. Hao, C. Wang, T. Wang, Chem. Phys. Lett. 443 (2007) 342.
M. Risti´c, S. Musi´c, M. Ivanda, S. Popovi´c, Journal of Alloys and Compounds 397 (2005) L1.
M. Tokuda in Novel Trends in Electroorganic Synthesis (1995) 241, S. Torii, Ed, Kodansha, Tokyo.
A. A Jalil, N. Kurono, M. Tokuda, Synlett. 12 (2001) 1944.
A. A Jalil, N. Kurono, M. Tokuda, Tetrahedron. Lett. 58 (2002) 7477.
A. A Jalil, N. Kurono, M. Tokuda, Synthesis. 18 (2002) 2681.
M. Tokuda, N. Mimura, T. Karasawa, H. Fujita, H. Suginome, Tetrahedron. Lett. 34 (1993) 7607.
M. Tokuda, N. Kurono, N. Mimura, Chem. Lett. (1996) 1091.
N. Kurono, K. Sugita, S. Takasugi, M. Tokuda, Tetrahedron. Lett. 55 (1999) 6097.
C. Hariharan, Appl. Catal. A 304 (2006) 55.
R. Wahab, S. G. Ansari, Y. S. Kim, H. K. Seo, H. S. Shin, Appl. Surf. Sci. 253 (2007) 7622.
Q. Xiao, S. Huang, J. Zhang, C. Xiao, X. Tan, J. Alloys. Compd. 459 (2008) L18.
H. Kleinwechter, C. Janzen, J. Knipping, H. Wiggers, P. Roth, J. Mater. Sci. 37 (2002) 4349.
L. Wu, Y. Wu, W. LÜ, Physica. E 28 (2005) 76.
U.N. Maiti, Sk.F. Ahmed, M.K. Mitra , K.K. Chattopadhyay, Materials Research Bulletin (2008) Article in Press.
H. Wei, Y. Wu, N. Lun, C. Hu, Mater. Sci. Eng. A 393 (2005) 80.



















